- Get directions
- Leave a review
- Claim listing
- Bookmark
- Share
- Report
- prev
- next
- Monday, May 5, 2025 @ 11:00 am
The Swiss biotech industry can rely on its independent, competent and internationally networked agency for therapeutic products. Collaboration with other national regulators strengthens Switzerland’s position as an innovative research and development location, and enables it to play a leading role in shaping global standards.

Jörg Schläpfer
Swissmedic | Head of Staff and External Relations
International networks are essential to the continued success of Swissmedic, the Swiss Agency for Therapeutic Products. Medicinal products and medical devices are developed and produced on a global basis, and new technologies and findings must be integrated into the regulatory environment. The increasing complexity of products and supply chains, as well as cost pressure, make it difficult for even large therapeutic products authorities to fulfil their mandate on their own, and cross-border exchange is crucial to ensure patient safety in line with the latest scientific findings. For this reason, Swissmedic works closely with other regulatory authorities and is actively expanding partnerships.
For a small authority such as Swissmedic, a clear strategic approach to international collaboration is crucial in order to seize opportunities and successfully overcome challenges. Swissmedic’s international cooperation is based on three pillars: harmonization of standards and procedures; information exchange and collaboration; capacity building and technical support. This commitment not only promotes public health by giving patients rapid access to innovative therapies and medical services, but also ensures efficiency and transparency in the development and authorization of therapeutic products – worldwide.
Helping to develop and unify global standards
Dozens of different vernaculars, imposed as divine punishment, led to the Tower of Babel failing due to a lack of mutual understanding. There were a similar number of “languages” involved in the authorization of medicinal products, but the importance of harmonization was recognized at an early stage. In 1990, regulatory authorities from Europe, the US and Japan, together with the pharmaceutical industry, set themselves a common goal of harmonizing the technical requirements for the authorization of medicinal products. The resulting initiative, the International Council on Harmonisation (ICH) based in Geneva, is a success story. The Common Technical Document (CTD/eCTD) has established a global standard that eliminates the former need for often tedious restructuring of authorization dossiers for different countries. The result: significantly less administrative work for researching companies.
As a Standing Regulatory Member of ICH and currently also as Vice-Chair of the ICH Assembly, Swissmedic contributes to the further development of global standards for the evaluation and authorization of medicinal products. This includes, for example, guidelines for conducting clinical studies (Good Clinical Practice, GCP), regulations for the manufacture of medicinal products (Good Manufacturing Practice, GMP) and authorization processes. Terminology that was also developed by the ICH, the Medical Dictionary for Regulatory Activities (MedDRA), even allows communication in a “common language”.
Working with partner authorities
International cooperation in the regulation of therapeutic products is conducted both bilaterally between individual authorities and multilaterally on various platforms.¹ These are either initiatives or private-law associations supported by several national therapeutic product authorities or international organizations.
Cooperation is an ongoing process at different levels. The first step is to build trust in the way the partners work, through the exchange of information and experience. In a next step, various forms of work sharing can be implemented, for example, by relying on the assessment results of partner authorities (see Figure 1).
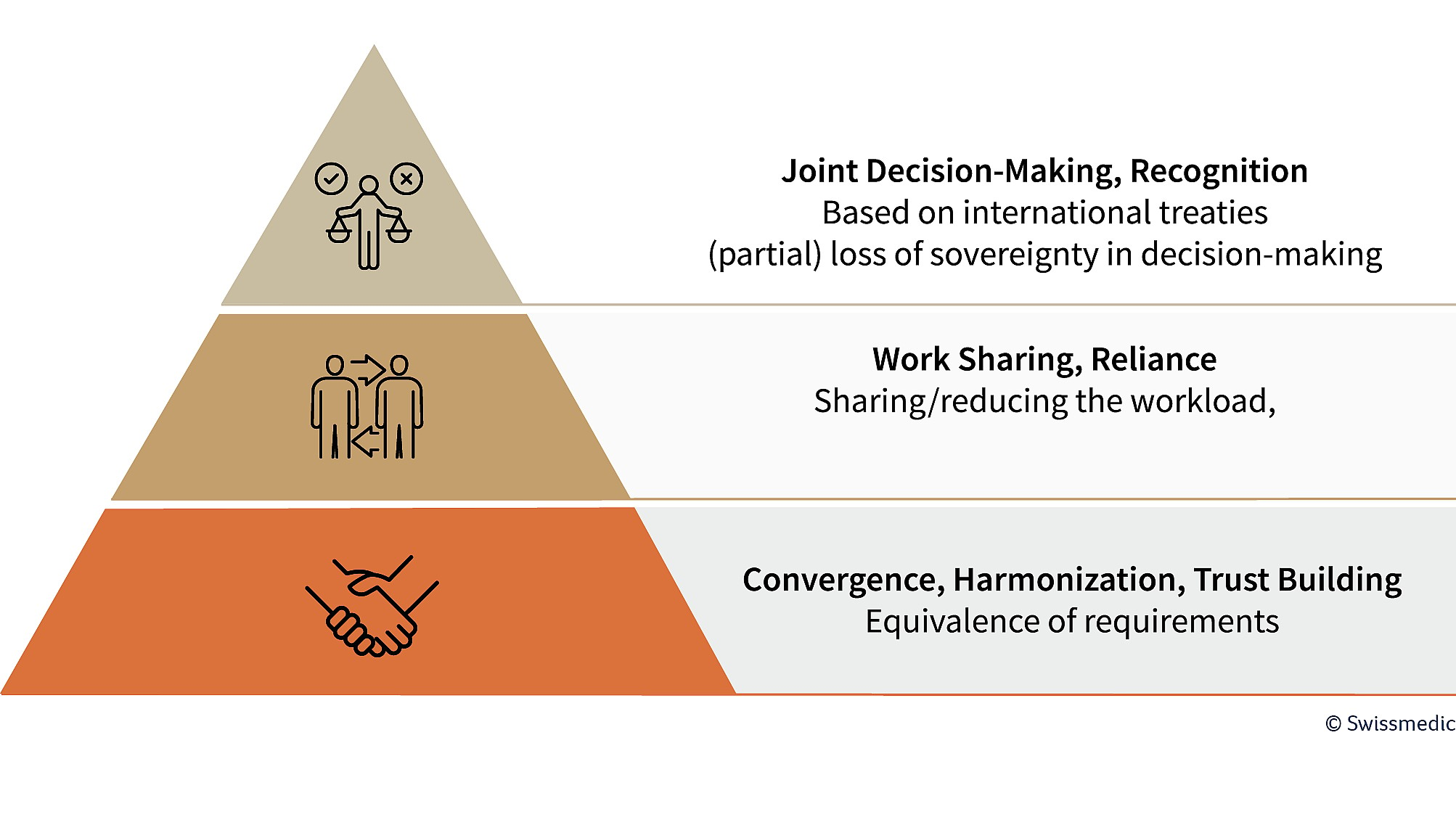
Swissmedic is involved in relevant bilateral collaborations and international networks on this basis
The interaction works like an orchestra: each authority contributes its expertise – comparable to a family of instruments. As part of work sharing, each authority takes on specific tasks, such as checking safety data or quality aspects. The joint “harmonious overall performance” results in benefit-risk decisions that can be accepted or rejected by the individual authorities.
A successful example of this is the Access Consortium,² a partnership between the therapeutic products agencies from Australia, Canada, Singapore, Switzerland, and the United Kingdom, which covers around 145 million people. The aim of the consortium is to speed up authorizations for medicinal products through coordinated assessment.
Project Orbis,³ a program initiated by the US FDA, also reflects the strength of multilateral cooperation. In this project, authorization applications for new cancer therapies are assessed in parallel by several therapeutic products agencies, in order to give patients transnational access to innovative cancer therapies as quickly as possible. The FDA oversees overall coordination, but each country involved remains completely independent as regards the final marketing authorization decision.
The optimized authorization processes for innovative medicinal products promote patient safety and are always carried out based on evidence needed for a risk-benefit assessment. This is one of the reasons why, in rare cases, the authorities involved may reach different decisions. The positive experiences with parallel review processes could help reduce the submission gap⁴ in other therapeutic areas outside oncology, thus enabling patients in Switzerland to gain faster access to innovative therapies.
These examples show how multilateral collaboration not only promotes the exchange of information and experience but also offers concrete solutions for efficiently managing the complexity of therapeutic product regulation.
Development cooperation: capacity building for sustainable health promotion
As part of the strategic Regulatory Systems Strengthening (RSS) program of the World Health Organization (WHO), Swissmedic supports countries with less developed regulatory systems through training and regulatory capacity building.⁵ This strengthens drug safety globally and promotes the harmonization of regulatory practices. In addition, Swissmedic has developed a procedure for scientific advice and Marketing Authorisation for Global Health Products (MAGHP procedure).⁶ This aims to speed up the authorization and market entry of high-quality, vital medicinal products for people in low-income countries, thereby contributing to improving global healthcare. The focus is on sub-Saharan Africa and on medicinal products for diseases that disproportionately affect this region. However, other countries or regions can also be considered.
The procedure provides a clear framework for the exchange of scientific and regulatory information between manufacturers and the regulatory authority. Pharmaceutical companies and manufacturers can seek scientific advice from Swissmedic at an early stage to ensure that their product meets all regulatory requirements for subsequent authorization in Switzerland and with the regulators involved. The authorization procedures offered comply with national and international standards.
National regulatory authorities are invited to actively participate in the evaluation with the aim of building up their own capacities and trust in the process. The first medicinal product authorized under the MAGHP procedure was an injectable medicine to prevent severe bleeding after childbirth. This approval, granted in May 2020, involved collaboration with National Regulatory Authorities (NRAs) from Uganda, Kenya, Tanzania (mainland and Zanzibar), South Sudan, Nigeria, the Democratic Republic of Congo, and Ethiopia.
Swissmedic is committed to international cooperation
Swissmedic is internationally renowned, well-networked and regarded as a competent, reliable partner. In this regard, the agency is guided by international standards. The ongoing harmonization of content-related and technical authorization requirements prevents duplications and speeds up the authorization process for high-quality, safe and effective medicinal products. This greatly benefits both patients and companies, ensuring that Switzerland remains an exceptionally attractive location for biotech companies.
References
1 www.swissmedic.ch/international
2 www.swissmedic.ch/access-consortium
4 Difference between the submission dates of applications to different authorities, in this case the time difference between the submission of an application for authorization to the reference authorities FDA/EMA and Swissmedic.
5 In 2023, Swissmedic was the first national regulatory authority to be classified as operating at Maturity Level 4 on the WHO Benchmarking List (www.who.int/initiatives/who-listed-authorityreg-authorities)